For food, beverage, petroleum, and other companies that handle liquids, flow rate and viscosity are two elements that affect an operation’s success and profitability. Whether it’s a production line enrobing chocolate-covered candy bars or an oil platform pumping up crude oil, production and profits drop when liquids become too viscous (thick).
The best way to increase and maintain the correct viscosity is to add heat. Temperature is the controlling factor for a better flow. A higher temperature lowers a liquid’s thickness, increasing the flow.
To control the thickness of fluids for consistency, we need to understand how to calculate it. This article will discuss its measurement, the units of viscosity, and ways to apply heat for production in any industry.
Powerblanket drum and barrel heaters are designed to provide optimal, uniform heating to maintain proper viscosity across various liquid products. Our heaters are custom-engineered with your specific container dimensions and product needs in mind.
What is Viscosity?
Most of us describe a liquid as thick or thin, but the technical term is viscosity. It represents a measurement of the resistance of objects passing through a fluid. The different layers of molecules in a liquid provide resistance as they try to move past each other.
Thicker liquids resist movement or shear stresses more than low viscosity liquids. For instance, if you drop a steel ball into a glass of water, it will sink faster than if you drop it into the same glass filled with honey.
In a production environment, it’s critical to know the viscosity of a liquid so that it will flow properly through the equipment. Too thick and spray nozzles can clog, too thin, and the product may run, overflow, or cause other problems.
Calculating viscosity will help you control it and know when a product is ready to use.
If you need more information about using formulas, go to our blog, “Let It Flow: How to Calculate Viscosity.”
How To Find the Units of Viscosity
Let’s review a few terms before getting into the formulas. In physics, there are two measuring systems. One is the Systeme International or SI system, which uses Meters, Kilograms, and Seconds or MKS units of viscosity for length, mass, and time. The other is centimeter, gram, and second (CGS) or Gaussian system.
Centipoise (cP) – A viscosity unit in cgs. It is 1/100th of the CGS unit named poise (P)
Poise (P) – A CGS unit of viscosity of dynamic viscosity. It’s pronounced “pwahz” after the French scientist Jean Léonard Marie Poiseuille.
To clarify, 1 centipoise is 0.01 poises and 1 poise is 100 centipoises.
Pascal-second (Pa·s) – A derived metric SI measurement unit of dynamic viscosity. Pa·s is equal to N × s ÷ m2 or kg/m/s. If you place a fluid between two parallel plates, the top plate pushes parallel to the bottom plate with a constant pressure of one pascal. If the top plate moves the same distance as the distance between the plates in one second, then the liquid between the plates has a dynamic viscosity unit of one pascal-second.
Stokes (St or S) – A fluid with a dynamic viscosity of 1 poise and a 1 gram per cubic centimeter density has a kinematic viscosity of 1 stokes. In SI units, one stokes equals 1 centimeter squared per second. However, the U.S.no longer uses a stokes unit of viscosity. Square cm per second is the preferred measurement.
Two Types of Fluids
There are two types of liquids when calculating viscosity: Newtonian fluids and non-Newtonian.
Newtonian Fluid – A Newtonian fluid’s viscosity remains constant and independent of the shear stress applied to them regarding time. The shear stress of these fluids is linear. Examples of these are water, alcohol, mineral oil, and gasoline.
Non-Newtonian Fluids – The opposite of Newtonian fluids, the viscosity changes when applying shear stress. Examples include toothpaste, ketchup, cosmetics, and paint.
The physics of viscosity can get more complex, but we’ll try and keep it as simple as possible.
Viscosity Formula
Shear Stress ÷ Shear Rate = Viscosity works for most industrial purposes. The viscosity unit is centipoise (cP), which is the equivalent of 1 mPa-s (millipascal second). You can try this Viscosity calculator if you require more detailed information.
Dynamic Viscosity vs. Kinematic Viscosity
There are two types of viscosity in physics: Dynamic or Absolute viscosity and Kinematic viscosity. Dynamic viscosity measures a fluid’s resistance to flow when applying pressure. It’s a calculation or ratio of shear stress to shear strain, measured in centipoise (cP), and Pa-s is the viscosity measurement unit. Dynamic viscosity provides information on the force needed to make the fluid flow at a particular rate.
Kinematic viscosity is the ratio of dynamic fluid viscosity to its density. It measures the flow resistance under the weight of gravity. Kinematic viscosity shows how fast the fluid flows when applying a specific force. In SI units, dynamic viscosity units are mPa-s, and the most common kinematic viscosity units are cm2/s.
Regarding centistoke units for kinematic viscosity, the stoke (St) is equal to 1 cm2 per second. Most measurements use the centipoise (cP) and centistoke (cSt), equivalent to 1/100th of the corresponding whole unit. An easy way to convert from kinematic to dynamic viscosity is to multiply the value in centistokes by the fluid’s specific gravity to get the corresponding value in centipoise.
Measuring Viscosity of a Liquid
One way to measure the viscosity of a liquid is to fill a graduated beaker, drop in a ball and measure the time it takes to fall to the bottom.
Here is the three-step process and formulas to do it.
Step 1 – Calculate the Density of the Ball
- Measure the mass of a ball using a balance. The ball’s mass is 0.1 kilograms (kg) in this example.
- Calculate the ball’s radius by first measuring the diameter (distance of a straight line through the ball’s widest part). Dividing the diameter by two calculates the ball’s radius.
- Determine the ball’s volume by adding its radius to the equation for the volume of a sphere. If the ball’s radius is 0.01 meter (m), then the volume calculation is: Volume = 4/3 x pi x (0.01 m) ^3 = 0.00000419 m3
- Next, determine the ball’s density by dividing its mass by its volume. The density in this case would be: Density = 0.1 kg ÷ 0.00000419 m3= 23,866 kg/ m3
Step 2 – Calculate the Density of the Liquid
- Measure the mass of an empty graduated cylinder. Then measure its mass with 100 milliliters (mL) of liquid. For this example, the empty cylinder’s mass is 0.2 kg, and its mass is 0.45 kg with fluid.
- Determine the fluid’s mass by subtracting the mass of the empty cylinder from its mass with the fluid. In this case: Mass of liquid = 0.45 kg – 0.2 kg = 0.25 kg
- Calculate the fluid’s density by dividing its mass by volume. Example: Density of fluid = 0.25 kg ÷ 100 mL = 0.0025 kg/mL = 0.0025 kg/cm3 = 2,500 kg/m3*
1 mL is equal to 1 cm3 *1 million cubic centimeters equal 1 cubic meter
To learn more information, go to our article, How to Calculate Viscosity.
Step 3 – Calculate the Viscosity of the Liquid
- Fill a tall, graduated cylinder with the test liquid about 2 cm from the top of the cylinder. Mark a line 2 cm below the liquid’s surface and another line 2 cm from the bottom of the cylinder.
- Measure the distance between the two marks. For this example, the distance is 0.3 m.
- Release the ball on the surface of the liquid and time how long it takes for the ball to drop from the first mark to the second mark.
- If the ball took 6 seconds to drop the distance, calculate the ball’s velocity by dividing the distance it fell by the time.
In this case:
The ball’s velocity = 0.3 m ÷ 6 s = 0.05 m/s
Using your data, calculate the viscosity of liquid:
Viscosity = (2 x (ball density – liquid density) x g x a2) ÷ (9 x v), where:
- g = gravitational acceleration = 9.8 m/s2
- a = radius of a ball bearing
- v = velocity of a ball bearing through liquid
To calculate the viscosity of the liquid, add your measurements to the equation. The calculation should look like this:
Viscosity = (2 x (23,866 – 2,500) x 9.8 x 0.012) ÷ (9 x 0.05) = 93.1 pascal seconds.
Dropping the ball through the fluid and timing its descent works, but there is an easier and more accurate way to measure viscosity.
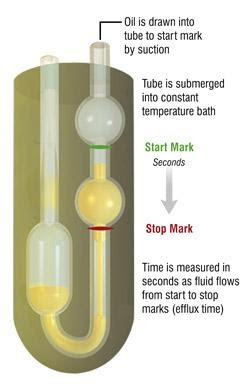
U-Tube Viscometer
U-tube viscometers are also called glass capillary or Ostwald viscometers. They consist of two reservoir bulbs and a capillary tube. The capillary in one arm of the U is a thin piece of tubing with a reservoir. On the other side of the U is two bulbs with a bit of tubing in between. The entire unit is inside a constant temperature water bath. Using suction or gravity, measuring how long one bulb takes to fill the other measures the viscosity.
Viscometers vs. Rheometers
Viscometers measure the viscosity of Newtonian fluids like water, air, alcohol, and thin motor oils. A rheometer uses a rotating disc lowered onto the sample to measure the viscosity of non-Newtonian fluids like grease at different temperatures.
Knowing the ideal thickness for a given liquid and how to measure it is essential in handling fluids for a process. The next step is to control it using heat.
To learn more, download the viscosity guide.
Controlling Viscosity with Temperature
The flow rate of a fluid is inversely proportional to the viscosity. As liquids get thicker, the slower the flow.
We can control a fluid’s viscosity by applying heat to lower it or chilling it to make the liquid thicker. There are two forces at play in liquid: cohesion and molecular interchange. When you shake a ketchup bottle to force it out of the opening, you affect the cohesion. When you heat a thick liquid like molasses or honey, the heat loosens the molecular interchange, allowing it to flow more freely.
Pounding on a 55-gallon drum to make it flow is impractical. Applying a heated blanket is the more sensible approach.
Viscosity in the Petroleum Industry
Measuring viscosity is critical to almost every process related to the petroleum industry. Examples include pumping drilling mud into the borehole for oil or gas, crude oil in pipelines, and motor oils in engines. Minor variations can significantly impact the properties of petroleum fluids.
A 1% error in a blend’s consistency can result in a cost increase of $0.01 per gallon. For oil companies, that’s millions of dollars in lost revenues.
Shear-Sensitivity in Food & Beverage Production
Most of our bulk processed foods start or end as liquid-like batters for bread and cakes. Beverages often require mixing syrups and flavorings of different densities to achieve a consistent product.
Production managers must maintain the same viscosity all year long to ensure batch consistency despite potentially different ambient and product temperatures.
Most liquid foods are “shear-sensitive.” Shear-sensitive liquids can change due to pressure and stress, such as the force of an impeller inside a pump. Some liquids become less dense, called shear thinning, while others become more viscous, called shear thickening or dilatant.
Related to viscosity is something called phase separation. If you shake a container with oil and water, the mixture quickly separates into its constituent components. Phase separation can happen in food processing where incorrect densities of ingredients cause the mix to break down and separate.
Cake batters for high-volume food factories must be sufficiently viscous to prevent the loss of gas bubbles incorporated during mixing. Gas bubbles help to reduce batter density and make the cake rise and become less dense after baking.
Temperature affects the batter’s viscosity. Once it’s in the oven, the heated batter becomes thinner, creating the possibility of phase separation. That means the flour and any solid fruits can sink to the bottom of the baking pan, resulting in an uneven cake, too dense on the bottom and light on top.
Food producers that use starch as a thickener, like soups, sauces, gravies, custards, etc., must maintain a consistent temperature to remain intact. Continued heating and shearing of starch (flour, corn, or rice) granules can break down, releasing water and reducing the viscosity, rendering the final product useless if not controlled.
Product Integrity in the Coatings Industry
Temperature control is essential for the coatings industry before dispensing. Industrial finishers include:
- Painting
- E-coating
- Anodizing
- Flow coating
- Roll coating
- Adhesives
- UV coating
- Potting
- Encapsulation
- Pump anti-condensation
- Bell anti-condensation
Maintaining the correct temperature affects the quality of the coating and finished product, either by reducing heat during the process or increasing it.
Heating & Cooling Methods to Control Viscosity
Liquids stored in warehouses may not be at the optimal temperature when delivered to the production line, and product temperature is a challenge for many industries.
Powerblanket offers various heating products for flow and viscosity solutions, from bucket and drum heaters to Intermediate Bulk Containers (IBC) tote heaters. We have portable and custom-designed industrial cooling systems for warehouses or processes.
Here are some of the viscosity maintenance and control solutions available.
Drum and Bucket Heaters
Keeping 5, 15, 30, or 55-gallon drums at constant temperature is challenging unless you use a drum heater. Drum heaters wrap around the entire drum, providing freeze protection, insulation, and continuous even heat.
Powerblanket Drum Heaters come with either a pre-set temperature or a programmable digital controller. These heaters are ideal for maintaining product viscosity at temperatures of 80°F (27°C) to 145°F (63°C) in cold production facilities.
IBC Tote Heaters
Critical materials require stringent storage and usage temperature requirements regardless of the ambient temperatures of the warehouse or production area. Solve viscosity and temperature issues with a Powerblanket Tote Heater. These wrap-around, insulating heaters fit tote sizes from 250 gallons to 550 gallons. The flexible design fits snugly, preventing hot and cold spots. They provide consistent heat even with ambient temperatures as low as -40°F/-40°C. These industrial heating blankets maintain viscosity for food products, paints, roofing materials, chemicals, epoxies, resins, equipment, and pallets.
Bulk Material Warmers
There is a simple, lightweight, and portable solution to maintaining a uniform heat of a pallet of 5-gallon buckets of temperature-sensitive products. Powerblanket Bulk Material Warmers are mobile solutions surrounding, covering, and insulating the entire pallet. It provides even heat, holding product temperatures between 100°F (38°C) and 120°F (49°C). For more accurate temperature control, order the optional adjustable thermostatic control. Accommodates pallets up to 4 ft x 4 ft x 5.5 ft.
DEF (Diesel Exhaust Fluid) Tote Heaters
DEF does an excellent job of reducing toxic NOx gasses in diesel engine exhaust. It’s typically a mix of 32.5% urea, or aqueous ammonia solution, and 67.5% deionized water. However, the water can freeze in winter weather and disrupt the flow. Patented Powerblanket DEF Storage Heaters preheat DEF and protect it from freezing down to -20°F (-28°C). Available for 275 gallons and 330-gallon totes plus DEF tanks installed on commercial trucks.
Bee Blanket
Bees and beekeeping are vital to all agriculture, providing pollination plus the delicious benefit of honey. Honey becomes a highly viscous fluid at lower temperatures, and raw honey must remain below 100°F (37.7°C). Powerblanket Bee Blankets provide even heat for uniform flow during processing and packing. Bee blankets will fit 5-gallon pails with or without valves, 55-gallon drums, and 36x48x48 palates or totes.
Wax Blanket
Wax is a staple ingredient in lipstick, cosmetics, food coatings, and candles, to name a few. Melting wax can be tricky. Some waxes can melt starting around 75°F (23.8°C), which affects the products stored in warm areas. Heating wax above 200°F (93.3°C) can discolor it brown, gray, or fade its color. Excess heat can cause a separation of the wax from any additives.
To heat wax to the proper, consistent temperature, Powerblanket developed the Wax Blanket. The heating elements embedded in the blanket heat a 5-gallon container uniformly from the bottom edge to the top edge. It’s the safest way to heat wax without babysitting or guesswork.
Gas Cylinder Warmers
Cold weather can reduce the viscosity of propane and pressure in the tanks. Low pressure prevents the gas from vaporizing, jeopardizing various production processes. The answer is to wrap propane tanks with a Powerblanket Propane Tank Heater. Adding heat along the entire tank surface reduces costs by optimizing temperatures and increasing propane tank efficiency. Available heaters fit propane tanks from 20 pounds to 1,000 pounds with fixed thermostats set to 100°F (38°C).
Temperature Stabilization Solutions
The most effective way to control the flow rate and viscosity of liquid products or ingredients is temperature. Preheating products to a set temperature reduces production time and improves consistency. Whether you need to increase or decrease heat or chill inside 5 gallons to the entire process, Powerblanket experts can help design the right solution.
Frequently Asked Questions
What is viscosity and its unit?
Viscosity is a measure of a fluid's resistance to flow, with its SI unit being the pascal-second (Pa·s) for dynamic viscosity and square meters per second (m²/s) for kinematic viscosity.
What are the two units of viscosity?
The two primary units of viscosity are the pascal-second (Pa·s) in the SI system for dynamic viscosity and the poise (P) in the CGS system, with kinematic viscosity measured in square meters per second (m²/s) or stokes (St).
What is the most common unit of viscosity?
The most common unit of viscosity in the CGS system is the poise (P), often used in its smaller form, the centipoise (cP), which is equivalent to 1 millipascal-second (mPa·s).
What is viscosity measured in?
Viscosity is typically measured in pascal-seconds (Pa·s) for dynamic viscosity and square meters per second (m²/s) for kinematic viscosity, with centipoise (cP) being a common alternative unit.
Powerblanket's industrial drum & barrel heaters provide even and consistent heating, eliminating waste and lowering costs.